sCD14-ST – A Novel Biomarker for Infection
Mindray is dedicated to providing inflammation total solution which consists of IL-6, PCT and sCD14-ST to meet different clinical demands from our end-users.
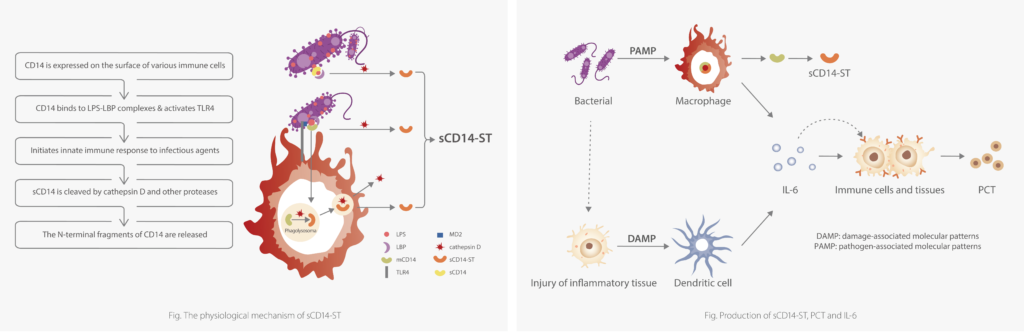
sCD14-ST (the soluble subtype of CD14 ) or presepsin, is a glycoprotein fragment derived from monocytes and macrophage. It is an innate direct response biomarker of activation of immune cell responding towards an invading pathogen. Compared to PCT induced by cytokines after bacterial phagocytosis, sCD14-ST is a more direct infectious biomarker, which ismediated by pathogens.
Clinical values
Studies on pathological mechanism and clinical trials have revealed that sCD14-ST is important for the clinical managementof infectious diseases or related conditions, such as neonatal sepsis, prosthetic joint infection(PJI), febrile neutropenia(FN),sepsis and early infection in trauma.
Neonatal spesis
Diagnosis and risk assessment
Prosthetic Joint Infection (PJI)
Diagnosis and prognosis

Sepsis
Bacterial infection identification
Sepsis and septic shock diagnosis
Sepsis severity assessment
Treatment monitoring

Febrile Neutropenia (FN)
Early identification

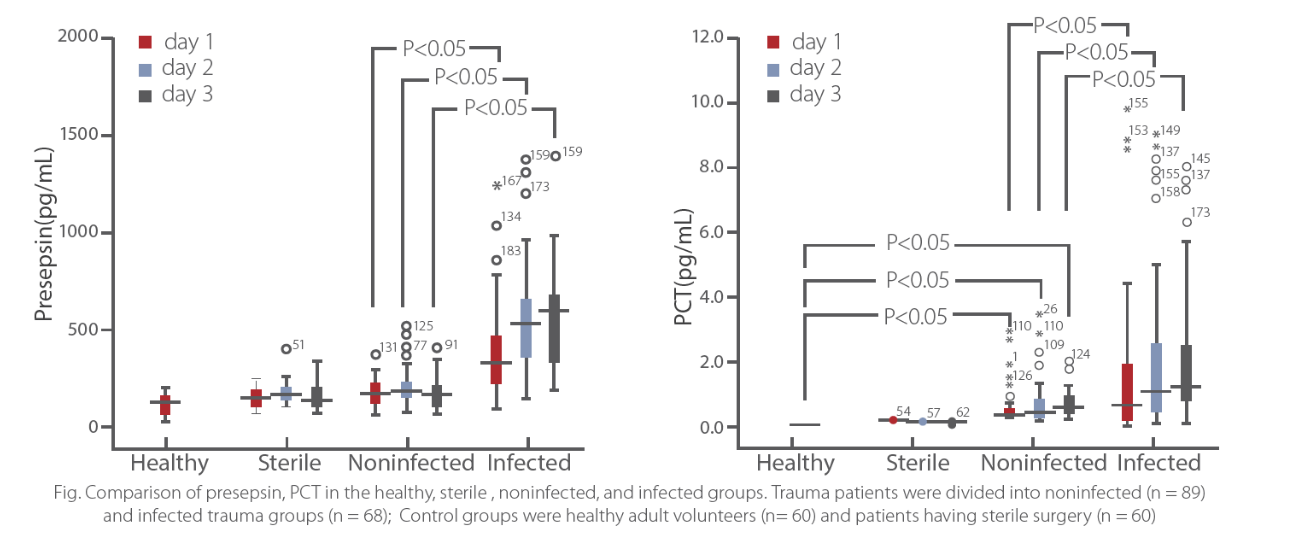
Trauma
Early differentiation
Neonatal Sepsis
sCD14-ST is a valuable biomarker for neonate sepsis, playing an important role in the rapid assessment and evaluation of severity. sCD14-ST levels in neonates with sepsis are signi cantly higher than those of the non-infective SIRS and normal control groups, suggesting that it could be an indicator for the identi cation of infective and non-infective SIRS.
sCD14-ST levels at T0 were significantly higher in neonates with sepsis and sepsis shock compared to those with infection. During the first 48h from the onset of symptoms, sCD14-ST progressively increased in neonates with septic shock, while it remained stable or decreased in neonates with sepsis or infection.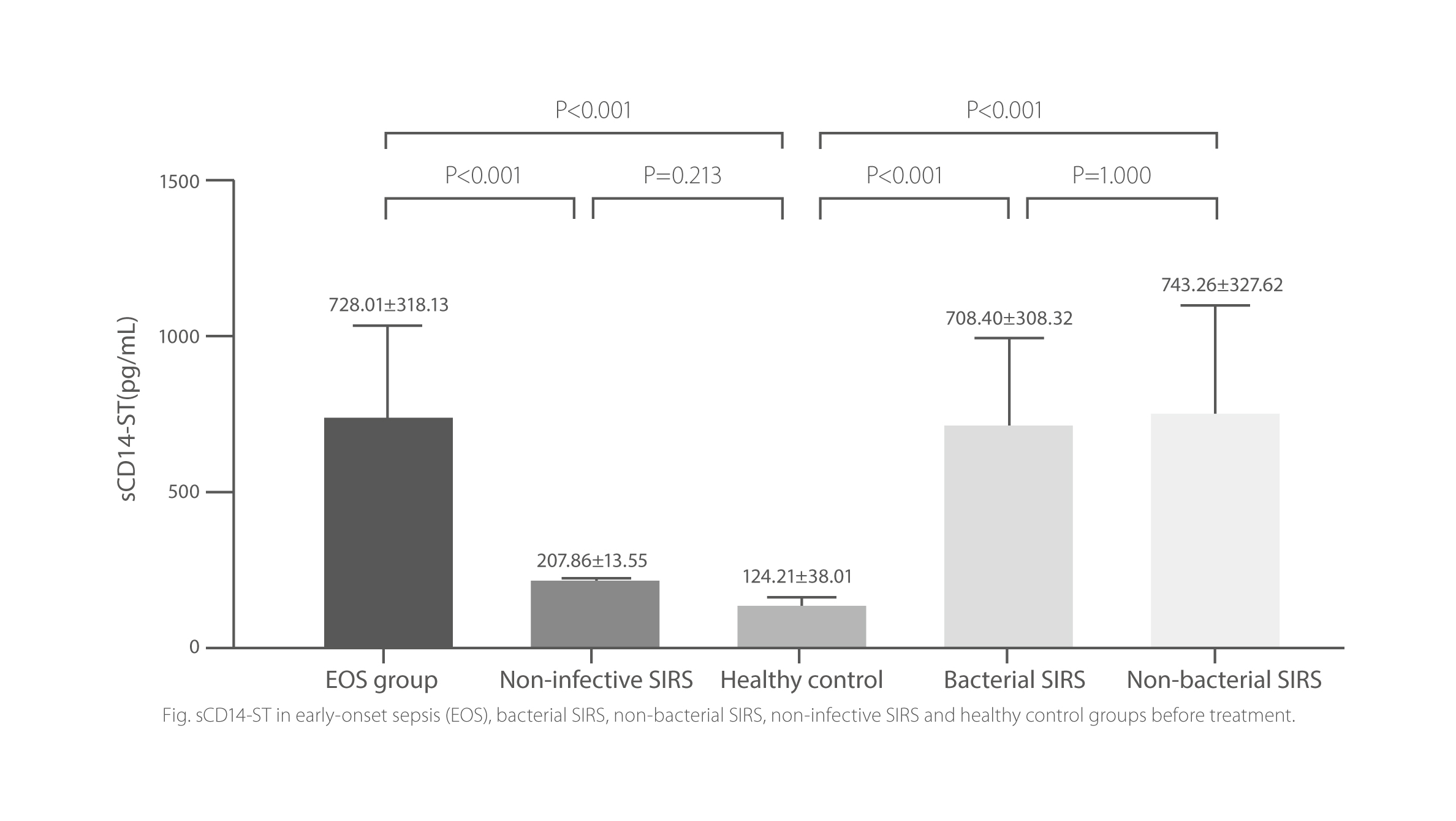
sCD14-ST in early-onset sepsis (EOS), bacterial SIRS, non-bacterial SIRS, non-infective SIRS and healthy control groups before treatment.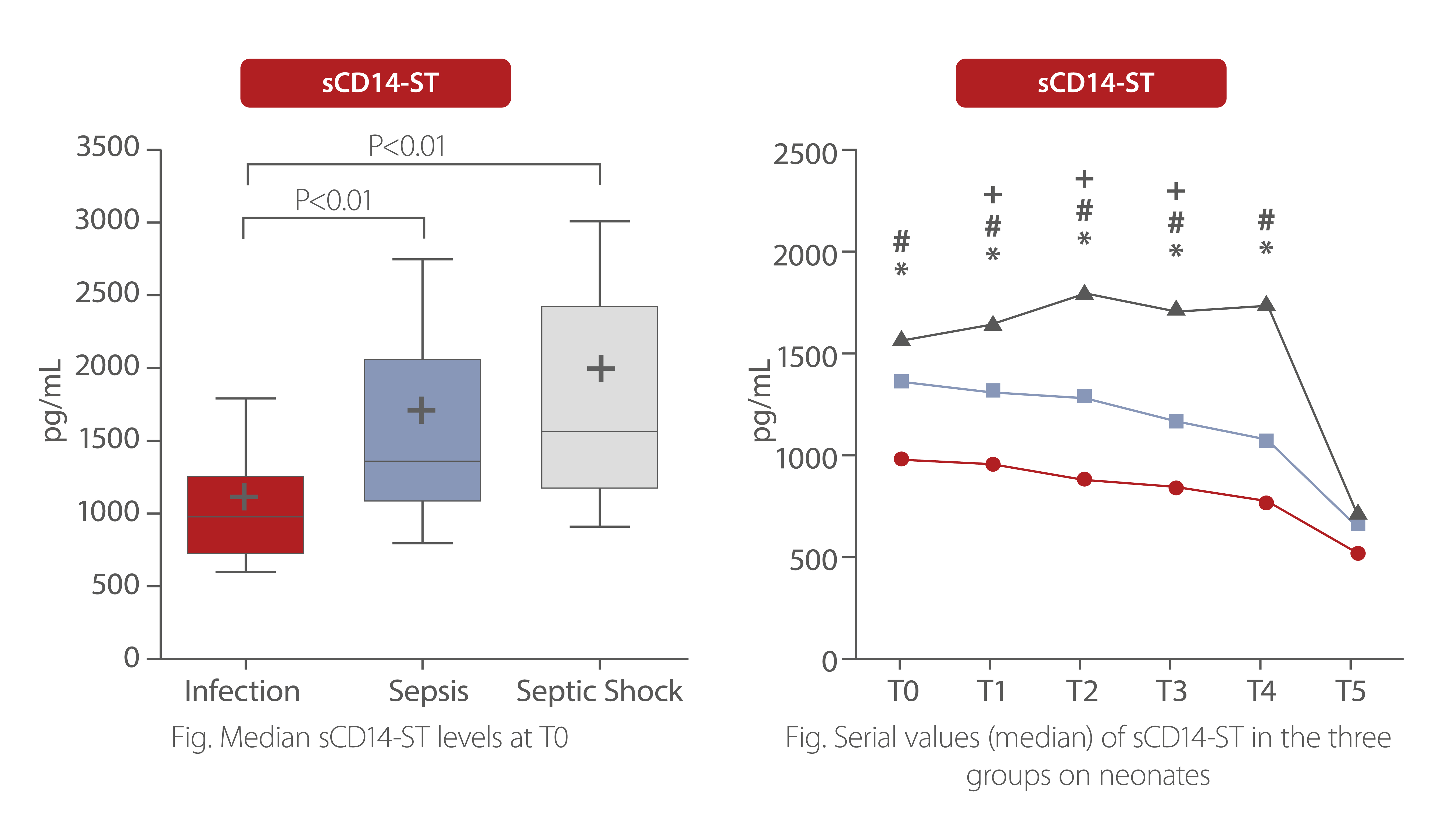
P<0.01 marked as follow: *, infection vs. sepsis; #, infection vs. septic shock; +, sepsis vs. septic shock T0: onset of symptoms. T1: 12h, T2: 24h, T3: 36h, T4: 48h, T5: end of antibiotic therapy


Prosthetic Joint Infection (PJI)
sCD14-ST is a potential inflammation biomarker for the diagnosis and prognosis of PJI. Research has shown that sCD14-ST levelswere significantly higher in PJI patients than controls. Post-operative levels of sCD14-ST in PJI patients dropped significantly with longer recovery time, while sCD14-ST levels remained unchanged and significantly lower in non-infected patients.
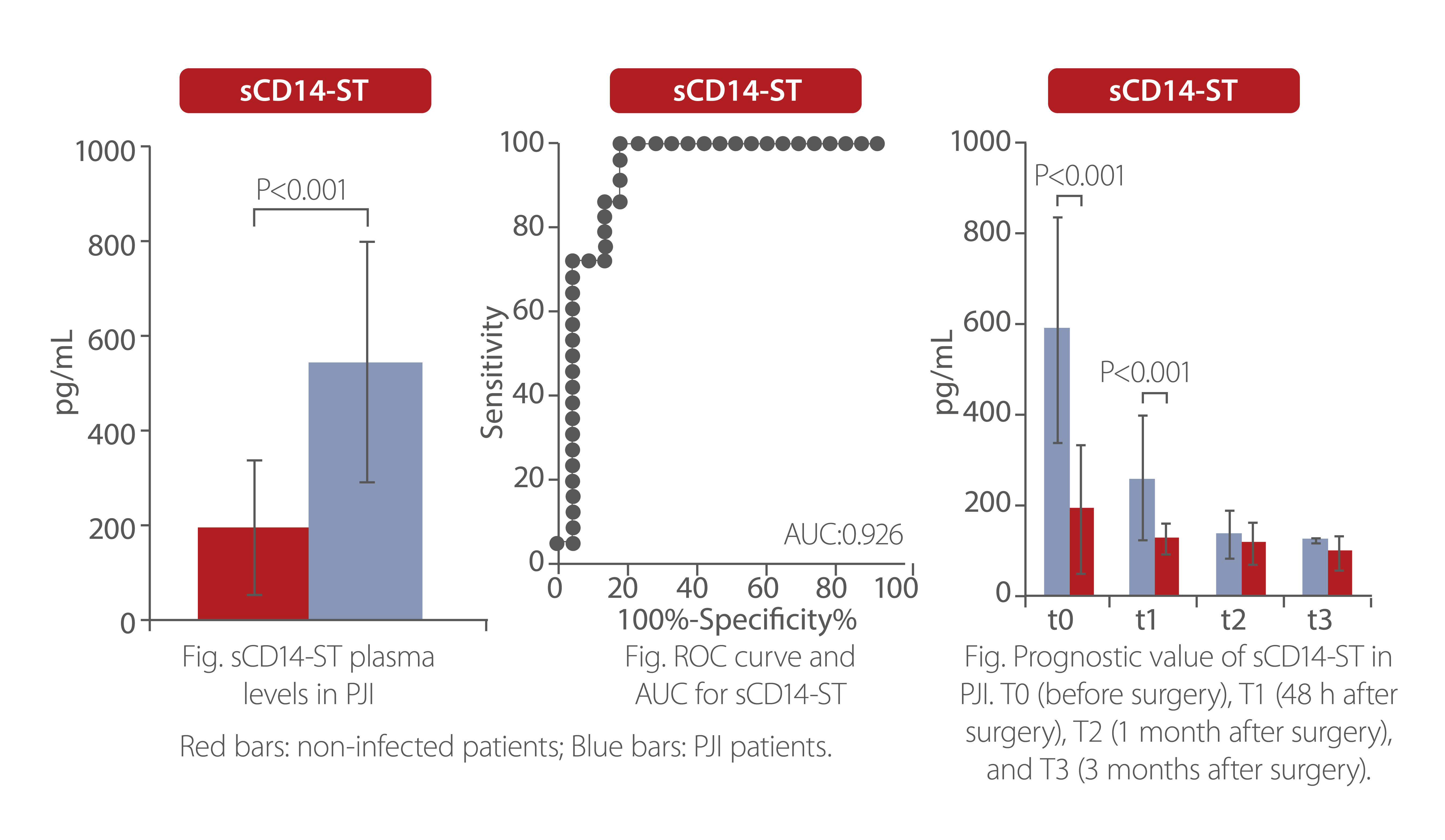
Febrile Neutropenia (FN)
sCD14-ST is an early diagnostic marker of febrile neutropenia in hematologic malignancy patients. In a related case study,elevated levels of sCD14-ST was observed one day prior to CRP. Plasma sCD14-ST level is a reliable marker of FN even incases of extremely low WBC counts. Further, evaluation of increase rate can facilitate early diagnosis of FN in patients with myeloid and lymphoid disorders. Closer monitoring of this molecule could prevent infection-associated death inhematologic malignancy cases.
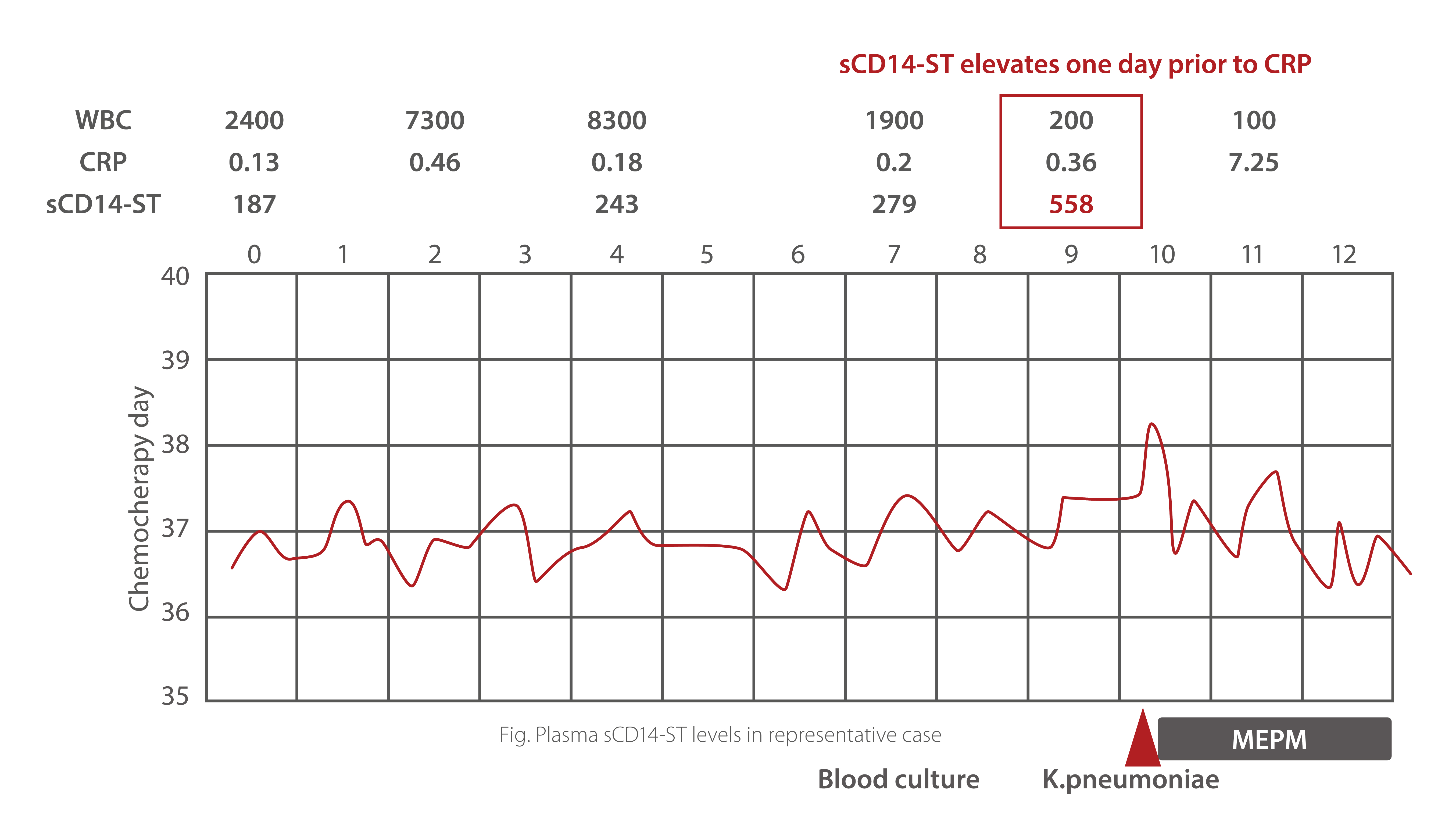
Sepsis
Biomarker for bacterial infection
sCD14-ST is an accurate biomarker for bacterial infection. The concentration levels of Mindray sCD14-ST in bacterial infection disease group are significantly higher than those in non-bacterial infectious disease and healthy groups.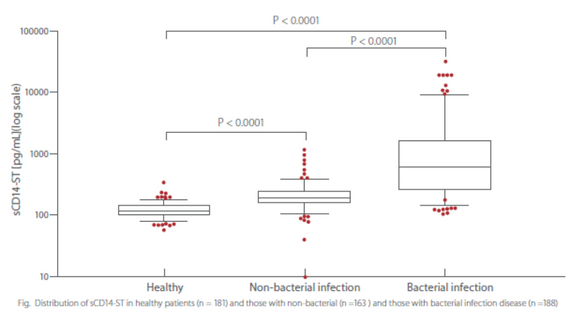
Predictive marker for adult sepsis
sCD14-ST is a predictive marker for adult sepsis. The level of Mindray sCD14-ST is significantly increased in patients with sepsis and septic shock, compared with those in bacterial infection and heathy groups. ROC analysis of sCD14-ST indicated good diagnostic accuracy showed a good diagnosing efficacy between ICU patients without sepsis and ICU patients with sepsis.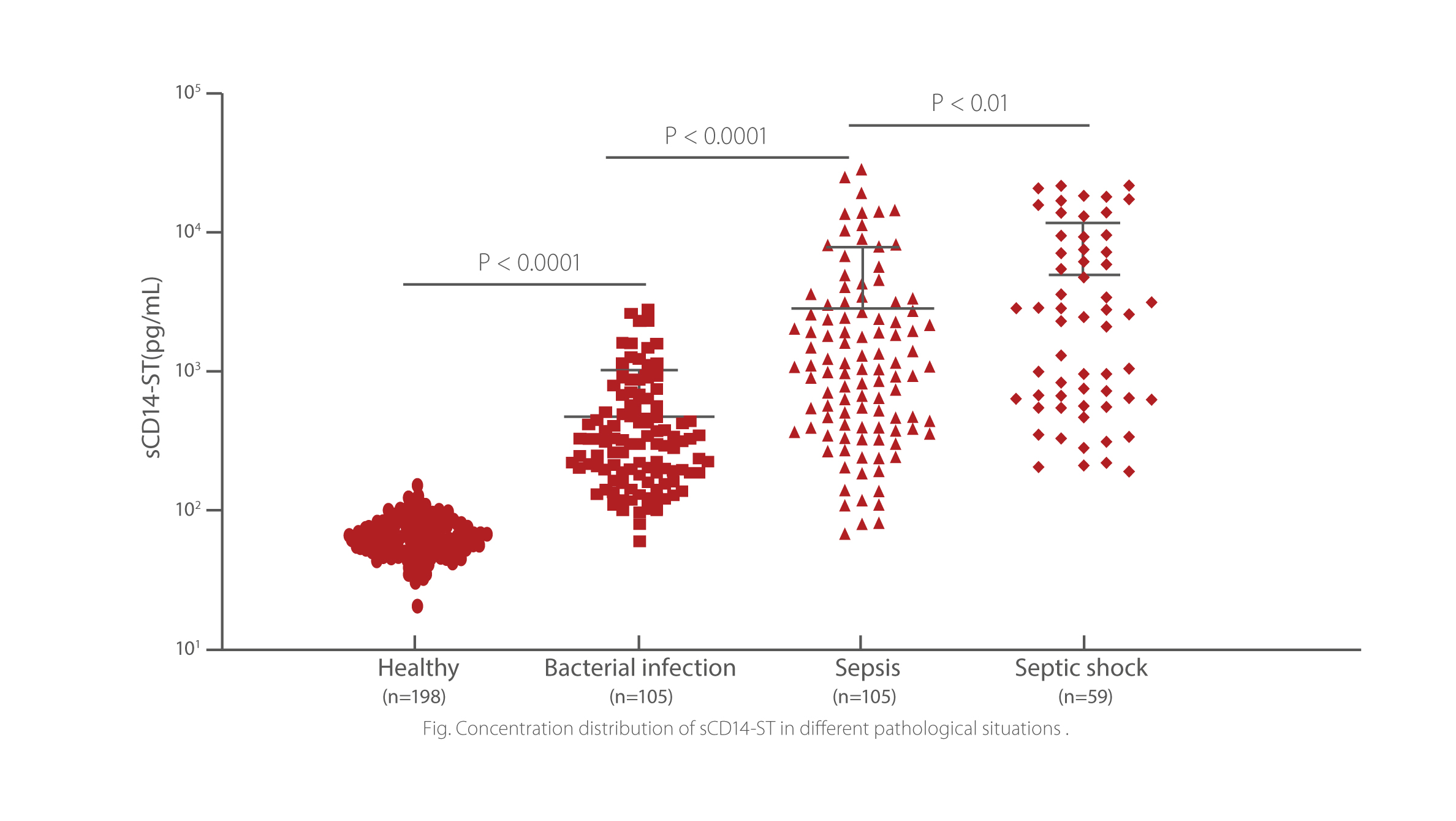
Sensitive biomarker for the assessment of sepsis severity
sCD14-ST is a sensitive biomarker for the assessment of sepsis severity in ICU patients. In this study, sCD14-ST level presented significant difference in severely ill (APACHE II >15) and critically ill (APACHE II >15) groups, while PCT and IL-6l evels displayed no statistically significant difference between these two groups.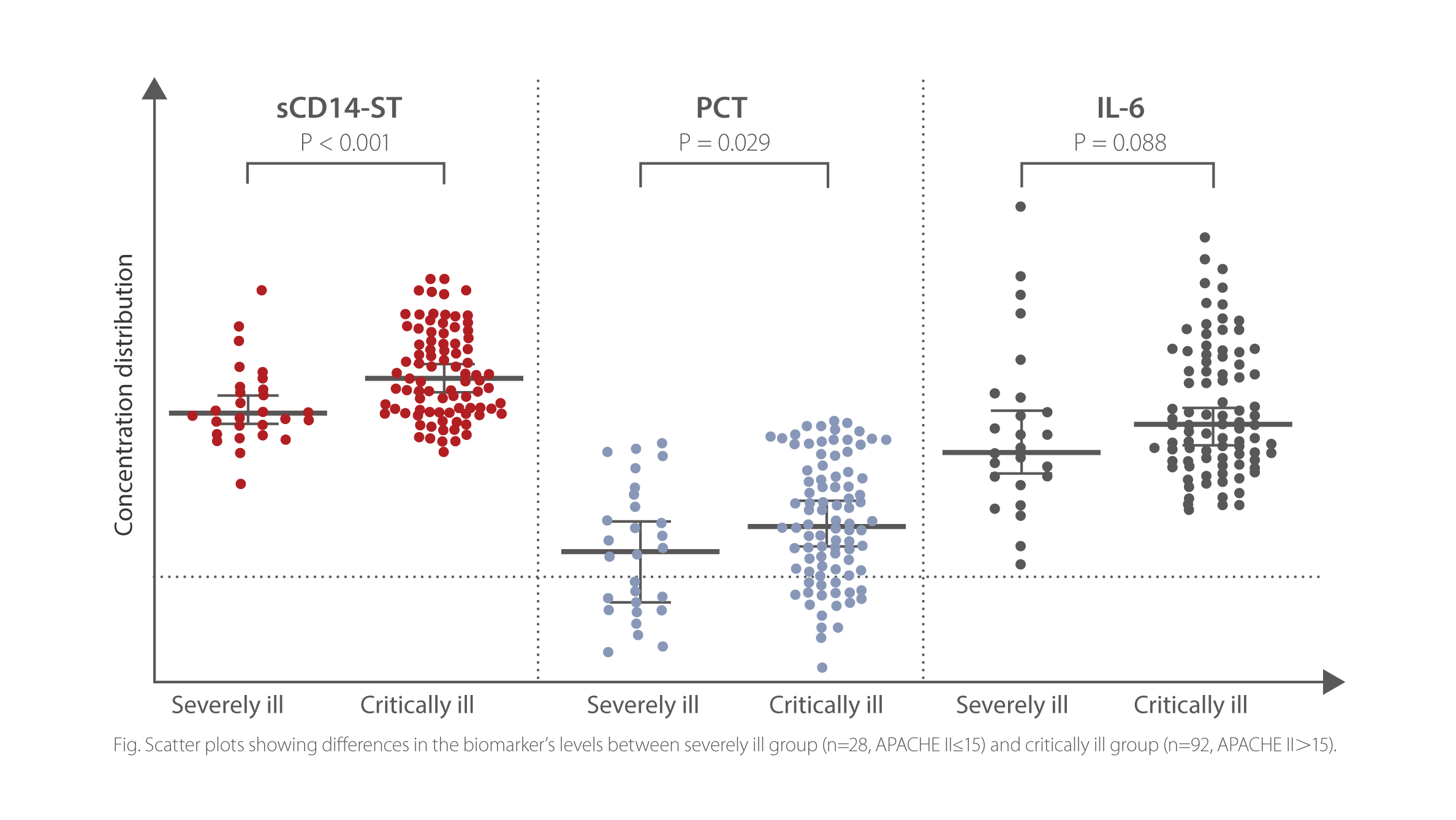
Powerful monitoring tool for the treatment of sepsis
sCD14-ST is a powerful monitoring tool for the treatment of sepsis. In the SOFA and APACHE II favorable group, sCD14-ST levels on D3 and D7 were significantly lower than levels measured at the time of admission. Meanwhile, in SOFA and APACHE II unfavorable group, sCD14-ST levels on D7 were not significantly lower than the levels measured at the time of admission.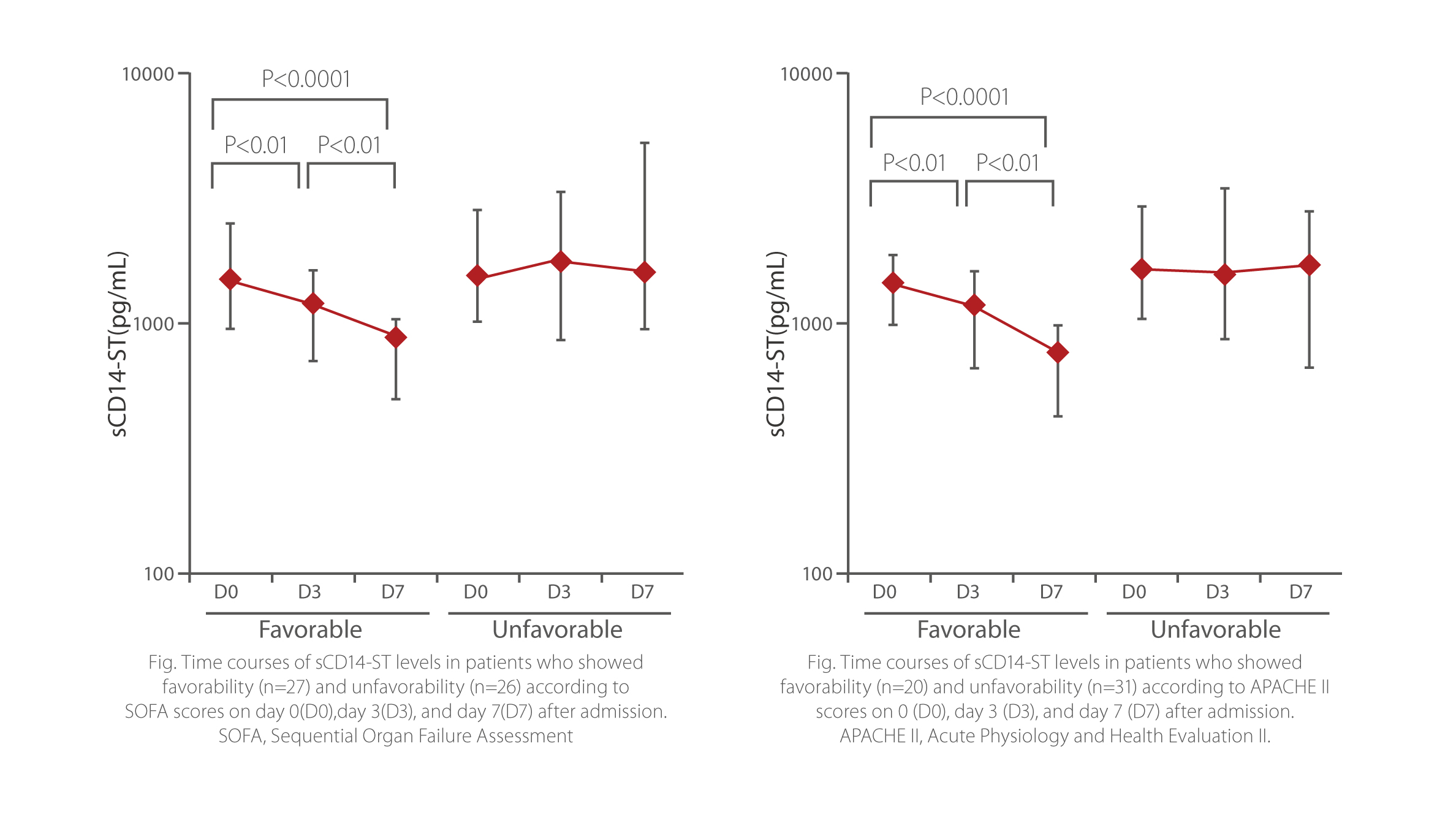


Traum
sCD14-ST is a superior biomarker for early differentiation of infection in trauma patients. Plasma sCD14-ST levels within the first 3 days of admission were only significantly increased in the infected trauma group, but not in the non-infected trauma and sterile group. sCD14-ST is also specified in the presence of infection in trauma patients.
Product Information
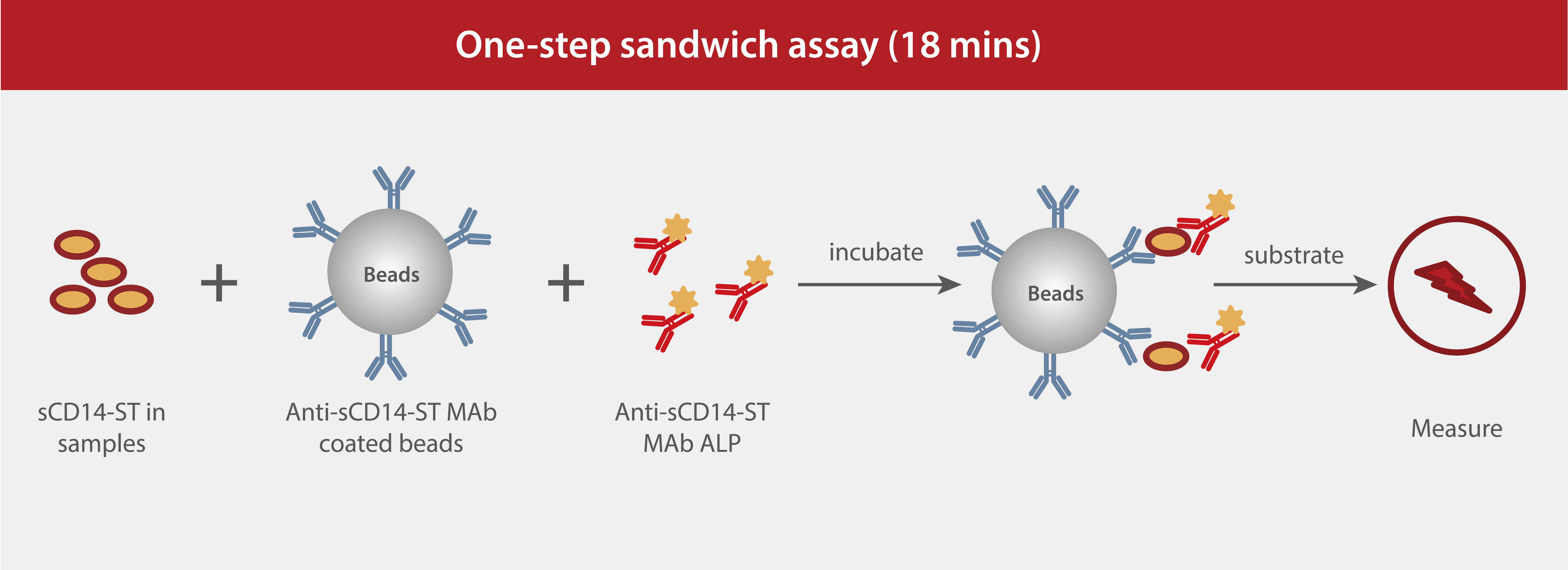
Mindray sCD14-ST assay principle
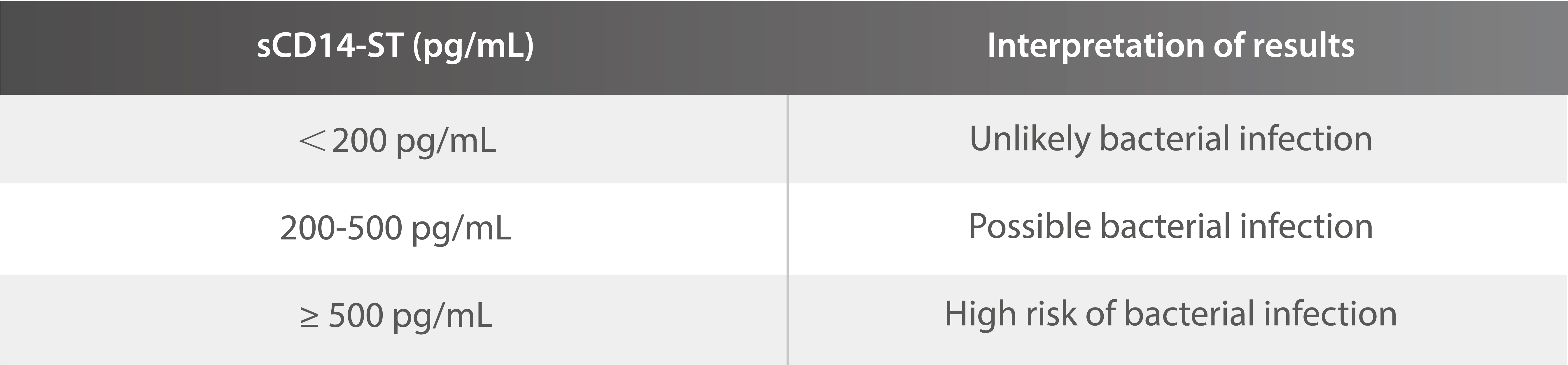
Threshold value and clinical significance
Order Information