Quality Slide Program (QSP 2.0)

Accreditation Support
Qualification tool for cytology staff in accordance with standard NF EN ISO 15189
The QSP 2.0 software is a tool for high-definition imaging, didactic and very intuitive. It offers to the laboratory staff the examination of blood films, which are scanned and appraised beforehand.
It allows the laboratory to assess the ability of prospective examiners to correctly identify individual WBC populations and other identifiable elements.
One of the essential steps in accreditation is the assessment of staff skills in accordance with Chapters 5.1.2.d (Assessment of Competencies) and 5.1.3 (Continuous Training) of the NF EN ISO 15189 standard.
To meet this requirement, HORIBA Medical has specifically developed for the cytology benchtop an innovative solution, accessible to all laboratories, to control the slide review.
QSP 2.0 helps to standardise an area of the laboratory where results may sometimes be subjective. Continuous improvements to FBC technology over the years has led to low blood film review rates, however this has resulted in the reduction of experience, staff confidence and subsequently a skills gap in haematology morphology review. QSP 2.0 helps to overcome these challenges by improving training in blood film morphology and providing a means of assessing staff competencies. The QSP 2.0 is available now for both demonstration and lunchtime presentations.
Are you looking for the QSP Monthly Newsletter? ►Click here to go the QSP Newsletter page
*This product may not be available in your country or region at this time. Please contact your HORIBA Medical sales representative or distributor for more information.
Segment: Medical
Division: Hematology
Manufacturing Company: HORIBA ABX SAS
- The subscription to the QSP 2.0 allows to receive 6 digitalized slides to be evaluated per month.
- Each clinical case, previously appraised, is validated by the referent of the laboratory.
- The virtual slides offer a minimum of 100 white blood cells to classify and allow the addition of comments on all blood populations and diagnostic analysis based on clinical information. To ensure perfect traceability, the QSP 2.0 delivers real-time customized reports and a synthesis of the entire laboratory with analysis of continuous improvement over time. These reports present complete performance statistics and gap analysis with the misclassified cells, giving to the lab a leading educational tool for continuous training.
- Now QSP2.0 network license is possible for up to 50 users and also it works on VPN/local networks.
- QSP2.0 is available in Single License format and Site License format.
- Reports are available in pdf and excel format.

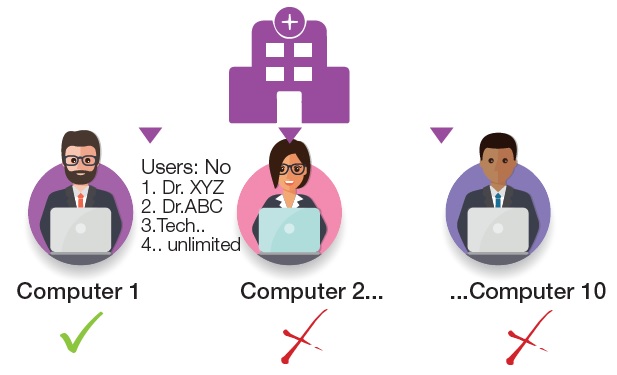
Single License
Option 1
License for an individual computer
Single computer license bound to a specific computer via a license file (software dongle, no physical USB dongle) Local installation of QSP2.0 with slide packages and user reports.
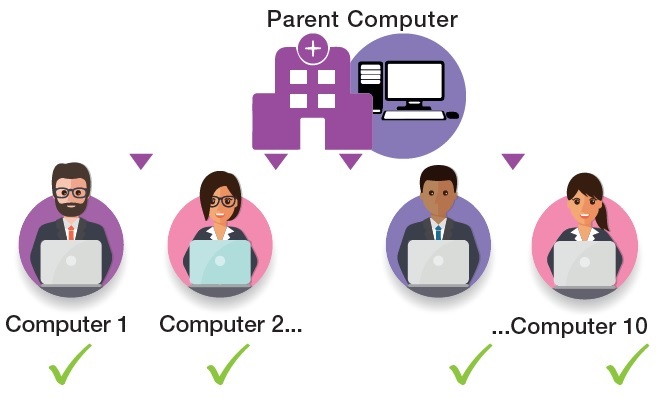
Site License
Option 2
License for up to 10 concurrent computers
Note for clarification: the site license is similar to individual QSP 2.0 installations with the added flexibility that it can be used on several computers where QSP 2.0 is installed and administrated. It is NOT a networked version of QSP 2.0, i.e. there is no shared database, integrated reports or automated installation of QSP 2.0 installations on all computers in the network.